I was once asked by my corporate executives to explain how cryopumps work with the orders, “Keep the physics out of it, Steve.”
“Hmmm…”, I thought to myself, “How the devil and I going to do that?” And there was

Cryopump figure from US6263679
the challenge. When dealing with the top executives, you don’t want to start the lecture with, “First there was a Big Bang…” and then talk about the history of the universe and how that lead to cryopumps. The executives were facing some technology challenges and they needed to understand actually what cryopumps are, what they do and why use a cryopump instead of a turbomolecular pump.
Then I realized that I although my execs are highly skilled engineers, they do not live and breathe vacuum technology day in and day out like I do. After all, that is why I am on the payroll, so they don’t have to put attention on the vacuum equipment. This post was inspired by the presentation that I put together for my execs, “Cryopumps for Layfolk”

We all have had the observation that frost and ice forms on a cold surface. Most of us were born after the advent of the frost-free refrigerators. Those of us who were not can remember the times when mom and dad had to defrost the freezer in the middle of summer when high humidity condensed and froze on the walls of the freezer. (I am in that latter mix.)
You may ask, “What does this has to do with vacuum technology?” That is a fair question.
Consider a closed chamber with a fixed amount air inside. That air exerts a pressure on the inside surface of the chamber.

Now let’s make the chamber wall so cool that the gas freezes on the side of the wall. Molecules that are taken out of the gas phase and made into a solid phase no longer contribute to the chamber pressure. 
This is the basic principle of cryopumping, use cold surfaces to capture molecules that are in a gaseous state so they do not contribute to the pressure in the chamber. There are three methods of capture.
The first method of capture is condensation, the process in which a gas changes state into a liquid, usually when it comes in contact with a cold surface. This is an excellent method of dehumidifying a room in the hot summer, but it is not very useful in high vacuum systems. Depending on the temperature, the liquid can easily revert back into a gaseous state and the pressure will remain relatively high.

In the chamber below we have a mixture of gases, hydrogen, Argon and water vapor. The total pressure in the chamber is a sum of the partial pressures of each of the gases. I have chosen these three gases because they are each captured differently in a cryopump, as you will see.

Now let’s put a cold surface in the system at 35ºF. This is cold enough to condense the water vapor, but the liquid water is still fairly mobile and will tend to drip in the chamber. We may expect a slight decrease in pressure. However the dripping in the chamber is not acceptable.

The second method is to capture gas with very cold surfaces. This is known as cryosorption. When a gas molecule strikes a cold surface and remains, it gives up heat to the surface. It can remain in solid form if the temperature is below the freezing point of the gas at the system pressure. This depends on temperature and pressure.
The way to capture gas and have it remain is to keep the surface temperature well below the freezing temperature of the gas.
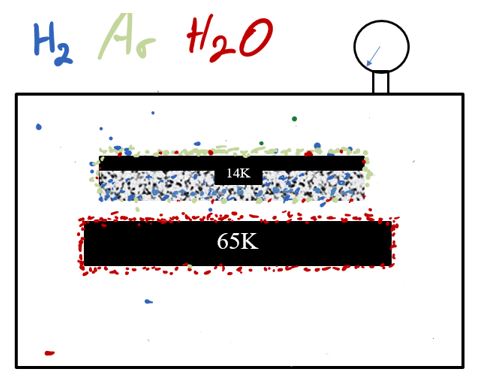
Let’s take our system and put a cold surface in that is well below the freezing point of water at the desired pressure. Here we have the surface chilled to 65K. At that temperature, nearly all of the water in the chamber that strikes the 65K surface will remain in solid form, thus it is no longer able to contribute to the pressure in the chamber and the pressure drops accordingly. A surface at 65K is cold enough to pump water vapor and many hydrocarbons.

The 65K surface is not cold enough to cryosorb H2 or Ar, so they will remain in a gaseous state. Some of the H2 and Ar may become trapped in the water ice, however a more efficient way to pump H2 and Ar is to put another surface into the chamber chilled to 14K.

The 14K surface is cold enough to freeze out the argon. It will also capture N2, CO, CO2, Kr, O2 and other such condensable gases. This leaves the non-condensable gases such as H2, He and Ne.
In our system, we still have the H2 gas remaining. There is a third cryopumping mechanism that is employed, cryotrapping. This method of pumping is done by taking a porous substance and chilling it down to a cryogenic temperature. When a gas molecule lands on the surface inside one of the pores, it will give up it thermal energy and reside there for an extended period of time. The colder the substance, the longer the gas will stay on the surface. In the days before dry roughing pumps, canisters of Zeolite were chilled in liquid nitrogen. The gas would then be cryotrapped in the high surface area Zeolite.
Another material is coconut charcoal. This has a high surface area and is extremely porous. Charcoal is typically cooled to between 9K and 15K for the purpose of cryotrapping H2. 
As the charcoal becomes saturated with H2, the ability of the charcoal to pump H2 will diminish. In order to rejuvenate the pump speed, the charcoal is warmed up and the hydrogen is liberated.
Now lets add a layer of porous charcoal on the 14K surface. Now we have a means to pump every gas that will be found in a vacuum system and the pressure drops accordingly.
Now that we know how to pump gases with cryogenic surfaces, we can take a look at the application of these methods.
 The construction of a cryopump is built around the cold-head cylinder. Inside the cylinder is a reciprocating “displacer” which is like a hollow piston which compressed helium is allowed to expand. There is a whole thermodynamic refrigeration cycle associated with this that is beyond the scope of this piece. I do have it explained in detail in the book Understanding Modern Vacuum Technology.
The construction of a cryopump is built around the cold-head cylinder. Inside the cylinder is a reciprocating “displacer” which is like a hollow piston which compressed helium is allowed to expand. There is a whole thermodynamic refrigeration cycle associated with this that is beyond the scope of this piece. I do have it explained in detail in the book Understanding Modern Vacuum Technology.
The figure to the left is from UMVT pg 206. Inside the cryopump, mounted to the refrigerator is the radiation shield. This surface is kept at 80K in this illustration and is set anywhere from 65K to 100K depending on the cryopump model. In my descriptions above, I used 65K to match our process requirements.
The radiation shield at 80K is thermally connected to the inlet 80K condensing array. Inside the pump, the top of the refrigerator is a cold stage that operates at 14K. An array containing bare surfaces and surfaces covered with charcoal is affixed to the 14K stage. Now let’s see how it all works together.

The cryopump is attached to a chamber. Water vapor in the chamber strikes the 80K frontal array and is cryosorbed onto the surface. Gases that do not cryosorb at 80K are allowed to enter into the pump. Gases such as Ar, N2, O2, etc. are cryosorbed onto the 14K surfaces and the He, Ne and H2 are cryotrapped in the charcoal. In most cryopumps, the charcoal is protected such that the condensable gases will strike a bare surface first, leaving the charcoal relatively free for trapping the non-condensable gases.
This particular design of a cryopump is used where there isn’t an appreciable amount of H2 in the chamber. It is easier for gases to strike the bare 14K surface first, which means the H2 has to make several collisions inside the pump before it becomes cryotrapped. This may reduce the H2 pump speed, which a compromise for this arrangement.
You may ask, “Why not just make everything 14K and be done with it?” There are two stages where arrays are attached to the cryopump refrigerator. The 80K stage (known as the first stage) can handle much higher amounts of the heat, 45 watts is a typical value. The 14K stage (know as the 2nd stage) can only handle on the order of 8 watts. Thus the 80K stage is used to handle the heat load from the external pump walls and the 14K stage only sees a 66K temperature difference rather than a 270K difference from the room temperature vacuum vessel wall.
There are many safety protocols that must be followed. Cryopumps are “capture” pumps, meaning that they store the gas pumped rather than compressing and exhausting it as in the cases of turbomolecular pumps and mechanical roughing pumps. Once the cryopump reaches capacity for a gas, the pump speed suffers. At that point the cryopump must be “regenerated” The pump is allowed to warm up and the gas is liberated. Unless the gas can escape, it can reach extremely high pressures at room temperature. In order to prevent the vacuum vessel from rupturing and creating a safety hazard, the gas is allowed to exhaust through a poppet valve that opens at just above atmospheric pressure.
Since a great deal of gas can be stored in the pump, proper safety precautions must be followed so that the ambient air is still breathable in the lab or reactive byproducts do not cause other hazardous conditions. Be sure to read and follow all safety information in the pump’s manual.
Finally, I have talked a great deal about pumping H2 with cryopumps. Processes involving H2 can create explosive conditions and there are a set of safety protocols specifically for H2. Of particular danger are the processes that produce ozone. Ozone is very reactive in cryopumps during the regeneration process. I bring this points up not to scare you away from cryopumps, I just want you to be aware.
I hope that you found this little primer helpful if you have not been exposed to cryopump technology before. In Understanding Modern Vacuum Technology, you will find a wealth of information about cryopumps, a detailed description of the closed loop helium refrigerator and information about cryopump safety.